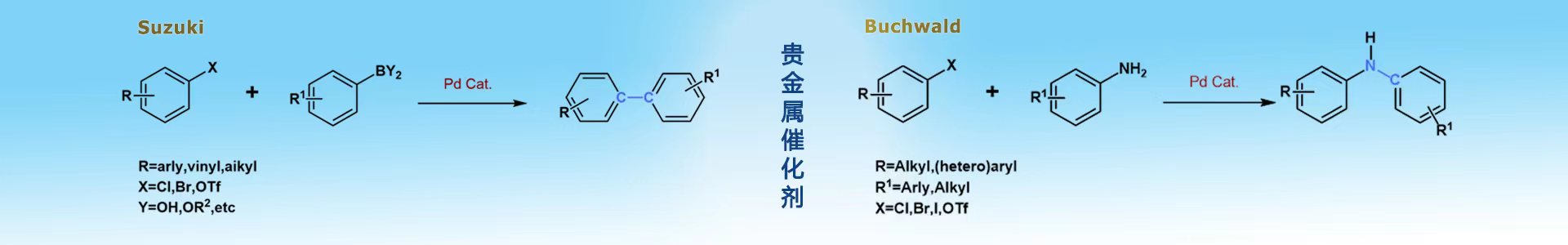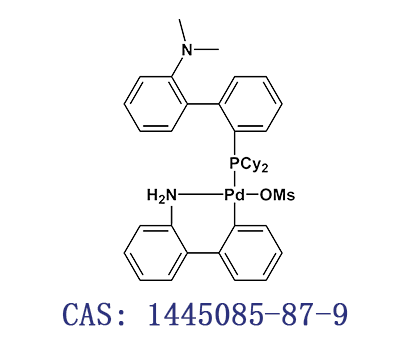
中文名称:甲烷磺酸(2-二环己基膦基-N,N-二甲胺基-1,1'-联苯基)(2'-氨基-1,1'-联苯-2-基)钯(II)
C A S号:1445085-87-9
分 子 式:C39H49N2O3PPdS
分 子 量:763.277
外 观:棕色固体
熔 点:183-200℃
纯 度:98%+
-
产品详情
第三代Buchwald预催化剂Davephos Pd G3,是各种交叉偶联反应的多功能催化剂。使用钯(0)预催化剂可实现与芳基卤化物与硼酸化合物的***效Suzuki-Miyaura反应交叉偶联。用于Buchwald-Hartwig构建C-N键。用于氨基化和烷氧基化反映。硼酸的氯磺酰化。合成tert-butyl4-(4-((4-(3-(2-fluoro-5-(hydroxymethyl)pyridin-4-yl)ureido)pyridin-2-yl)ethynyl)phenyl)piperidine-1-carboxylate(叔丁基4-(4-(4-(3-(2-氟-5-(羟甲基)吡啶-4-基)脲基)吡啶-2-基)乙基)苯基)哌啶-1-羧酸酯)、1-(2-fluoro-5-(hydroxymethyl)pyridin-4-yl)-3-(2-((3-formylphenyl) ethynyl)pyridin-4-yl)urea(1-(2-氟-5-(羟甲基)吡啶-4-基)-3-(2-(3-甲酰苯基)乙基)吡啶-4-基)尿素)。可用于制备环氧合酶(COX)(例如,环氧合酶2 (COX2))抑制剂化合物。这些化合物可以用放射性标记,所述化合物(如放射性标记化合物)可用于诊断疾病(如正电子发射断层显像剂),这些化合物也可能对治疗或预防疾病有用。
相关资料
1. Chem. Eur. J.26, 70,2020,16818-16823.https://doi.org/10.1002/chem.202003137.
2.Anal. Chem. 2021, 93, 4, 2610-2618.https://doi.org/10.1021/acs.analchem.0c04726.
3.Advanced Science,2022,9,27,2198-3844.https://doi.org/10.1002/advs.202202710.
4.Adv. Synth. Catal. 2016, 358, 1833-1847. https://doi.org/10.1002/adsc.201600173.
5.ACS Macro Lett. 2020, 9, 1357−1362.https://doi.org/10.1021/acsmacrolett.0c00580.
6. Isr. J. Chem,20, 60,1-10.https://doi.org/10.1002/ijch.201900170.
7.Org. Process Res. Dev. 2020, 24, 10, 1948-1954.https://doi.org/10.1021/acs.oprd.0c00018.
8.Applications of Transition Metal Catalysis in Drug Discovery and Development: An Industrial Perspective.DOI:10.1002/9781118309872.
9. Journal of the American Chemical Society (2016), 138(29), 9081-9084.
DOI:10.1021/jacs.6b05505.
10.WO 2013184198 A1.
11.WO 2021050700 A1.
12.WO 2020251971 A1.
13.WO 2018039232 A1.
14.WO 2017143348 A2.
15.WO 2013184198 A1.
16.Journal of the American Chemical Society, 135(29), 10638-10641; 2013
DOI:10.1021/ja405949a.
17.Journal of the American Chemical Society, 139(33), 11590-11594; 2017.
DOI:10.1021/jacs.7b06630.
18.CN 111971277 A
19.CN 110036001 B
20.Angewandte Chemie, International Edition, 54(28), 8259-8262; 2015.
DOI:10.1002/anie.201502626.