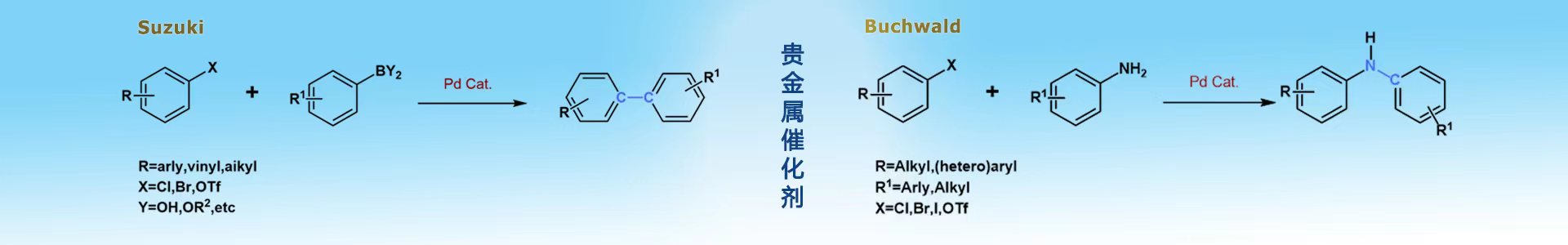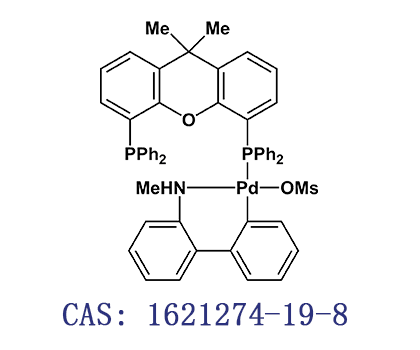
中文名称:甲烷磺酸(4,5-双二苯基膦-9,9-二甲基氧杂蒽)(2-甲胺基-1,1-联苯-2-基)钯(II)
C A S 号:1621274-19-8
分 子 式:C92H78NO8P4PdS2-
分 子 量:1620.050664
外 观: 类白色至浅黄色粉末
规 格:98%
-
产品详情
第四代Buchwald预催化剂Xantphos Pd G4,用于使用化学计量的CO从芳基溴化物和氟代硫酸盐中获取芳基和杂芳基三氟甲基酮的合成反应。用于合成(S)-1-氨基-1,3-二氢螺[茚-2,4'-哌啶]-6-甲腈、1-(6-氯-1-(四氢-2H-吡喃-2-基)-1H-吡唑并[3,4-b]吡嗪-3-基)-1,2,3,4-四氢-1, 5萘啶、喹啉-5-甲腈、3-[[(叔丁氧基)羰基氨基)-7-氰基-1,3-二氢螺[茚-2,4'-哌啶]-1'-羧酸叔丁酯、(R)-N-[(3S)-1'-(5-溴嘧啶-2-基)-1,3-二氢螺[茚-2,4'-哌啶]-3-基]-2-甲基丙烷-2-亚磺酰胺、(R)-N-((S)-1'-(6-溴-5-甲基吡啶-3-基)-1,3-二氢螺[茚-2,4'-哌啶]-1-基)-2-甲基丙烷-2-亚磺酰胺、(S)-(1'-(6-溴吡啶-3-基)-5-氟-1,3-二氢螺[茚-2,4'-哌啶]-1-基)氨基甲酸叔丁酯、(R)-N-((R)-1'-(6-溴吡啶-3-基)-3H-螺[苯并呋喃-2,4'-哌啶]-3-基)-2-甲基丙烷2-亚磺酰胺、N-[(3R)-6-氟-1'(吡啶-3-基)-3H-螺[1-苯并呋喃-2,4'-哌啶]-3-基]1,1-二苯基甲亚胺、1-{6-[(3S)-3-{[(叔丁氧基)羰基]氨基}-1,3-二氢螺[茚-2,4'-哌啶]-1'-基]-1-(氧杂环己烷 -2-基)1H-吡唑并[3,4-b]吡嗪-3-基}-1,2,3,4-四氢喹啉-6-乙酸酯、叔丁基 N-[(3)-1'-[5-(2,3-二氢-1H-吲哚-1基)-3-(羟甲基)吡嗪-2-基]-1,3-二氢螺[茚- 2,4'-哌啶]-3-基]氨基甲酸酯、叔丁基N-[(3S)-1'-[1-(氧杂-2-基)-3-(1,2,3,4-四氢-1,5-萘啶-1-基)-1H-吡唑 [3,4-b]吡嗪-6基]-1,3-二氢螺[茚-2,4'-哌啶]-3-基]氨基甲酸酯(tert-butyl N-[(3S)-1'-[1-(oxan-2-yl)-3-(1,2,3,4-tetrahydro-1,5-naphthyridin-1-yl)-1H-pyrazolo[3,4-b]pyrazin-6yl]-1,3-dihydrospiro[indene-2,4'-piperidin]-3-yl]carbamate)、叔丁基N-[(3S)-1'-{3-[(2,3-二氯吡啶-4-基)氨基]-1-(氧烷-2-基)- 1h -吡唑啉[3,4-b]吡嗪-6-基}-1,3-二氢螺旋体[茚-2,4'-哌啶]-3-基]氨基甲酸tert butylN-[(3S)-1'-{3-[(2,3-dichloropyridin-4-yl)amino]-1-(oxan-2-yl)-1H-pyrazolo[3,4-b]pyrazin-6-yl}-1,3-dihydrospiro[indene-2,4'-piperidin]-3-yl]carbamate、叔丁基((1S)-1'-(3-(3,4-二氢-1,5-萘啶-1(2H)-基)1-(四氢-2H-吡嗪-2-基)- 1h -吡唑啉[3,4-b]吡嗪-基)-1,3-二氢螺旋体[茚-2,4'-哌啶]-1-基)氨基甲酸酯tert butyl((1S)-1'-(3-(3,4-dihydro-1,5-naphthyridin-1(2H)-yl)1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazolo[3,4-b]pyrazin6-yl)-1,3-dihydrospiro[indene-2,4'-piperidin]-1-yl)carbamate中间体,这些中间体用于合成抑制SHP2磷酸酶活性的化合物以及组合物。介导一氧化碳和三氟甲基三甲基硅烷羰基化反应,从相应的(异)芳基溴和氟硫酸盐直接生成(异)芳基a,a-双(三氟甲基)甲醇。催化芳基磺烷氧羰基化反应。选择性4 -甲基咪唑N-布赫瓦尔德芳基化合成吡吡嗪-1,6-二酮γ-分泌酶调节剂。使用Buchwald Xantphos Pd G4预催化剂,通过选择性钯催化的4-甲基咪唑n -芳基化,构建结晶内酯中间体[合成1402004-16-3(7-(4-methyl-1H-imidazol-1-yl)-3,4-dihydropyrido[2,1-c][1,4]oxazine-1,6-dione、7-(4-甲基-1H-咪唑-1-基)-3,4-二氢吡啶并[2,1-c][1,4]恶嗪-1,6-二酮)],不需要预活化步骤,***效合成吡啶吡嗪-1,6-二酮γ-分泌酶调节剂(GSMs)。可扩展的二氧化碳电还原结合羰基化反应化学,合成药物吗氯贝胺。魏奈布酰胺前体的合成。选择单一的二溴化螺二芴作为 A-A 单体,并选择一系列芳族二胺作为 B-B 组分,使用XantPhos Pd G4钯环催化剂提供了螺二芴芳基二胺 (SBAD) 系列。合成3-Chloro-5-isopropyl-8-((2R,3S)-2-methyl-3-
(methylsulfonylmethyl)azetidin-1-yl)isoquinoline/3-氯-5-异丙基-8-((2R,3S)-2-甲基-3-(甲基磺酰基甲基)氮杂环丁烷-1-基)异喹啉,随后通过Buchwald-Hartwig偶联反应合成BLU-945,BLU-945是一种可逆的、有效的、保护野生型的下一代EGFR突变抑制剂,用于治疗难治性非小细胞肺癌。使用1.5当量的CO前驱体(COgen)和盐酸N,O-二甲基羟胺作为反应中间体,在钯催化剂(Xantphos Pd G4)的存在下生成Weinreb酰胺。芳基二甲基磺盐的烷氧羰基化反应。
相关资料
1.Org.Lett.2020,22,11,4068-4072.DOI:10.1021/acs.orglett.0c01117.
2.US10934302.
3.Ring walking as a regioselectivity control element in Pd-catalyzed C-N cross-coupling.https://doi.org/10.1038/s41467-022-30255-1.
4.Angew.Chem.130,23,June4,2018,6974-6978.https://doi.org/10.1002/ange.201802647.
5.Org.Lett.2019,21,8,2518-2522.https://doi.org/10.1021/acs.orglett.9b00067.
6.J.Org.Chem.2019,84,8,4921-4925.https://doi.org/10.1021/acs.joc.8b02953.
7.Scalable carbon dioxide electroreduction coupled to carbonylation chemistry.DOI:10.1038/s41467-017-00559-8.
8.Tetrahedron Letters Volume74,22 June 2021,153177.
https://doi.org/10.1016/j.tetlet.2021.153177.
9.Chem.Commun.,2021,57,5235-5249.https://doi.org/10.1039/D1CC01265G.
10.Org.Process Res.Dev.2017,21,9,1320-1325.
https://doi.org/10.1021/acs.oprd.7b00185.
11.POLYMER MEMBRANES FOR THE SEPARATION OF COMPLEX NATURAL HYDROCARBON FEEDS.
12.Org.Lett.2021,23,20,8001-8006.https://doi.org/10.1021/acs.orglett.1c03003.
13.Chem.Asian J.Volume15,Issue4,February 17,2020,441-449.
https://doi.org/10.1002/asia.201901644.
14.G3 and G4 Buchwald Precatalysts: Scale up, QC and application for the semi-automated parallel synthesis.
15.J.Org.Chem.2021,86,21,15674-15688.https://doi.org/10.1021/acs.joc.1c02251.
16.SCIENCE 17 Jul 2020 Vol 369, Issue 6501 pp. 310-315.
DOI: 10.1126/science.aba9806.
17.Process Economics and Atom Economy for Industrial Cross Coupling Applications via LnPd(0)-Based Catalysts.DOI: 10.1007/3418_2019_28.
18.J.Med.Chem.2022,65,14,9662-9677.https://doi.org/10.1021/acs.jmedchem.2c00704.
19.Funcionalização de 5-iodo-1,2,3-triazóis e síntese de seleno- e tioésteres por meio de reações de acoplamento carbonilativo empregando monóxido de carbono gerado ex situ.